CELLULAR DRIVERS OF REJUVENATION
Extensive meiotic organelle remodeling produces gametes that are devoid of cellular aging hallmarks (image from Sing et al, 2022)
Extensive cellular remodeling in the form of organelle morphogenesis, membrane biogenesis as well as protein and organelle degradation occurs during gametogenesis, yet we know very little about the underlying principles and regulation of these events. While each process itself inspires a number of fundamental biological questions, a broad understanding of this cellular restructuring program is especially important, given our finding that gametogenesis leads to elimination of senescence-associated factors. These include protein aggregates, dysfunctional organelles and extrachromosomal circular DNA (ecDNA). Gametes no longer contained any of these cellular defects that were present in the aged precursor cells.
Using live cell-microscopy and molecular genetic approaches, we obtain dynamic and mechanistic insights into the cellular remodeling processes that occur during meiosis. We are specifically interested in examining how nuclear physiology, mitochondrial dynamics and protein homeostasis are affected during meiotic differentiation.
Nuclear Remodeling and Selective Nuclear Inheritance
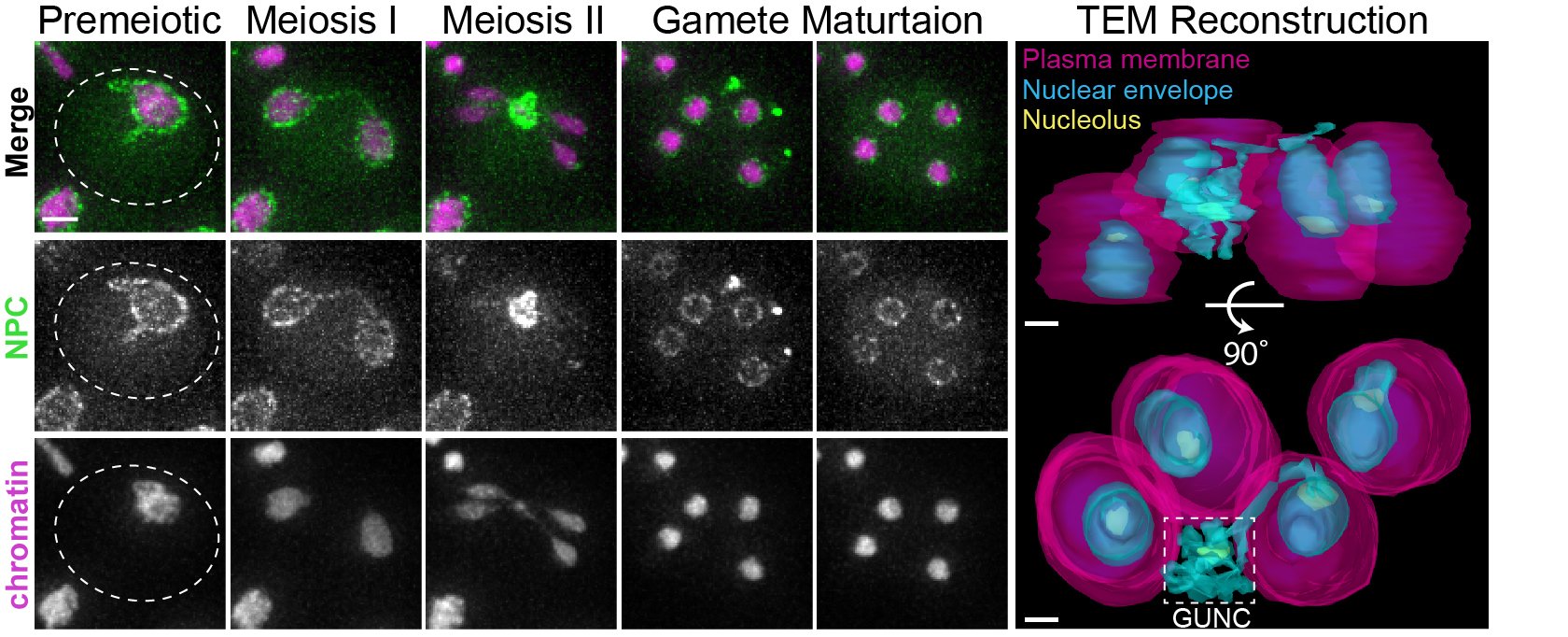
Senescence-associated nuclear factor are eliminated during gametogenesis through sequestration and degradation-based mechanisms (from King and Goodman et al., 2019).
We uncovered a dramatic nuclear restructuring event in meiosis, which involves the sequestration of nuclear pore complexes (NPCs) into a membranous compartment away from the dividing chromosomes destined for future gametes. We named this discarded structure GUNC, which stands for Gametogenesis Uninherited Nuclear Compartment (King and Goodman et al 2019; King and Ünal, 2020). Even though this event is an inherent part of meiosis (i.e. occurring in both young and aged cells), we found it to be essential for the successful elimination of age-associated nuclear defects, thus ensuring gamete rejuvenation. However, the mechanism by which NPCs are sequestered into the GUNC is still poorly understood.
Our findings thus far are consistent with a hypothesis that gamete plasma membrane development triggers the formation of a novel scaffold at the rim of growing membranes, which helps to organize a nuclear envelope diffusion barrier during gametogenesis, thereby culminating in selective nuclear inheritance and subsequent gamete rejuvenation. In order to identify this scaffold, we are undertaking an unbiased genetic screen .
Nuclear pore complexes (NPCs) are excluded from newly formed gametes (from King and Goodman et al., 2019).
Interestingly, a similar type of NPC remodeling occurs during metazoan spermatogenesis (Ho et al, 2010). Furthermore, several NPC subunits are exceptionally long-lived proteins, accumulating damage over time in yeast, worms and mammals (Sakuma & D’Angelo, 2017). These similarities across such diverse evolutionary lineages suggest an ancestral need to reorganize the nucleus during meiotic differentiation, perhaps for gamete rejuvenation. Thus, our studies could shed light into nuclear quality control pathways in metazoan gametogenesis.
Mitochondrial Remodeling, Inheritance and Quality Control
Transmission electron microscopy (TEM) of meiotic cells reveals extensive mito-nuclear contacts during the meiotic divisions.
Although a strong link between mitochondrial dysfunction and aging has been established, whether and how damaged mitochondria are identified and partitioned in meiosis remains elusive. Evidence suggests that mitochondrial selection occurs in the metazoan germline (Hill et al 2014; Tworzydlo et al 2016; Lieber et al 2019) , however the underlying mechanisms remain largely unexplored. Interestingly in budding yeast, up to 50% of the initial mitochondrial pool is partitioned amongst the four gametes (Brewer and Fangman,1980), while the remaining mitochondria are eliminated by megaautophagy, which involves the programmed lysis of the progenitor cell’s vacuole, an organelle equivalent to the mammalian lysosome (Eastwood et al 2012). Whether or not there are functional differences between the inherited and discarded mitochondrial populations is currently unknown. Shortly before meiosis II, mitochondria become localized near the nuclei, forming abundant contact sites with the nuclear envelope. An attractive hypothesis is that the mechanisms that mediate mito-nuclear tethering contribute to limited inheritance of mitochondria during meiosis and therefore could enable selective inheritance of healthier mitochondria.
Our data so far suggest that membrane contact sites between mitochondria and nuclear envelope are established by novel tethering components that are specific to meiotic cells. To identify the proteins responsible for mito-nuclear tethering, we are undertaking both genetic and biochemical approaches. Following characterization, we will disrupt the mito-nuclear tether(s) and determine how this perturbation affects propagation of healthy mitochondria and gamete rejuvenation.